Medical Equipment Electrical Safety Inspection

There are general and specific standards for medical device electrical safety.
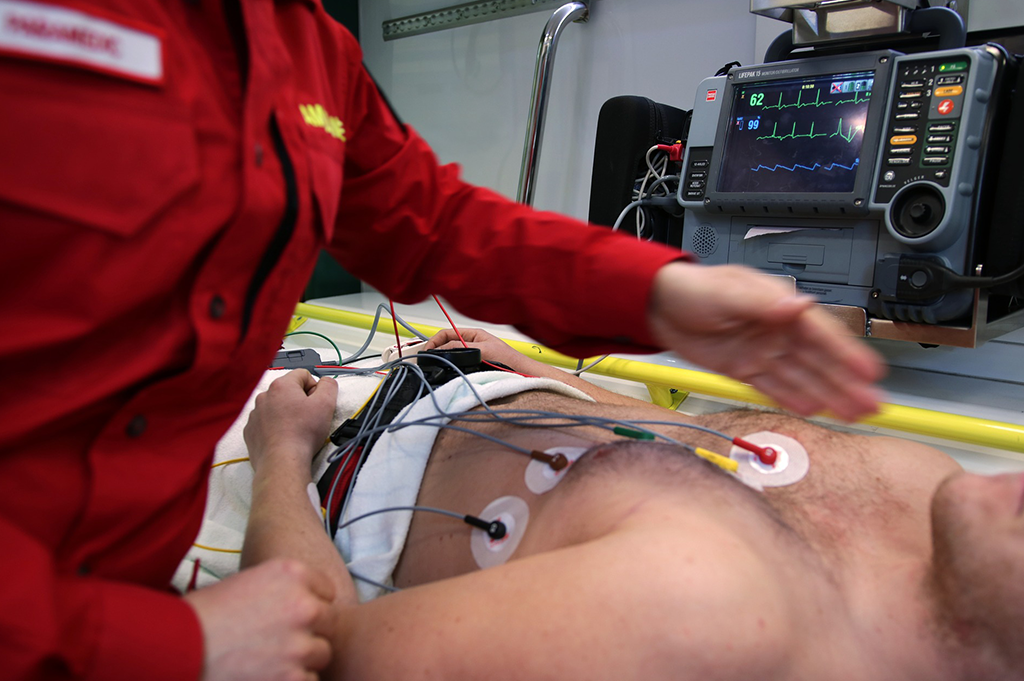
Medical equipment electrical safety inspection. The medical equipment management plan defines the mechanisms for interaction and oversight of the medical equipment used in the diagnosis treatment and monitoring of patients. These include standards for electro medical equipment. Patient treatment areas for all doctors physiotherapists dentists chiropractors and aged care facilities require specialised electrical work and stringent medical equipment testing which is compliant against as nzs 3003. Carelabs is authorized provider of electrical installation s study analysis inspection and certification services in uae and offer biomedical equipment safety testing and inspection services.
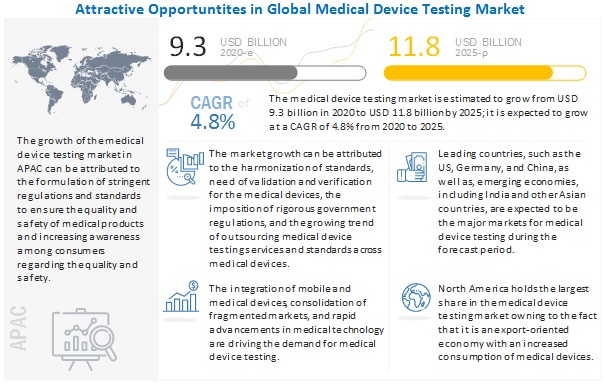
In addition accreditation and regulatory agencies. They should be integrated into a comprehensive equipment inspection and maintenance program for the facility. Special inspection service spe 3000 was developed to address the need for an evaluation of electrical safety of medical equipment and medical electrical systems. Iec 60601 is a series of technical standards for the safety and essential performance of medical electrical equipment published by the international electrotechnical commission first published in 1977 and regularly updated and restructured as of 2011 it consists of a general standard about 10 collateral standards and about 80 particular standards.
Adhering to manufacturer s recommendations for monitoring and maintenance of recording equipment. The plan must address monthly visual inspection of equipment by staff for apparent defects. Iec60601 aami nfpa 99 the primary standard for medical devices is iec 60601. Calmedinc has been in business for 30 years providing preventive maintenance calibration and electrical safety checks inspections for medical equipment.
Equipment in compliance with the requirements and bearing the special inspection service label is considered to be acceptable to the ahj. Call the experts today. Biomedical equipment armamentarium are equipment designed to aid in the diagnosis monitoring or treatment of medical conditions. General requirements for protection against electric shock hazards are covered in iec 60601 1 section 3.
It should be noted that electrical safety procedures for medical devices and healthcare environments are not intended to be separate from other equipment maintenance activities. Medical equipment testing inspection calibration.