Melting Point Of Natural Rubber

Importance of the melting point of rubber chemicals.
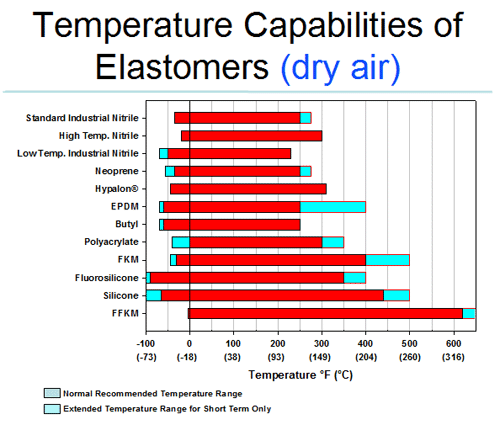
Melting point of natural rubber. Depending on its molecular structure polyisoprene can be a resilient elastic polymer as in the case of natural rubber and isoprene rubber or a tough leathery resin as in. Natural rubber is an elastomer and a thermoplastic. This rubber o ring temperature chart shows high and low rubber temperature range resistance for all popular materials. Polyisoprene polymer of isoprene c 5 h 8 that is the primary chemical constituent of natural rubber of the naturally occurring resins balata and gutta percha and of the synthetic equivalents of these materials.
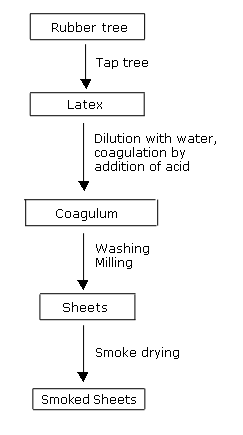
Make sure that your material is compatible with your environment by going to our rubber properties and chemical compatibility sections. The tree hevea brasiliensis secretes a milky substance called latex which is used to produce rubber. Some natural rubber sources such as gutta percha are composed of trans 1 4 polyisoprene a structural isomer that has similar properties. Most modern shoe soles are not rubber as in natural latex based but are some form of plastic heat will melt most thermoform plastic but the problem will be making a suitable mould for the sole.
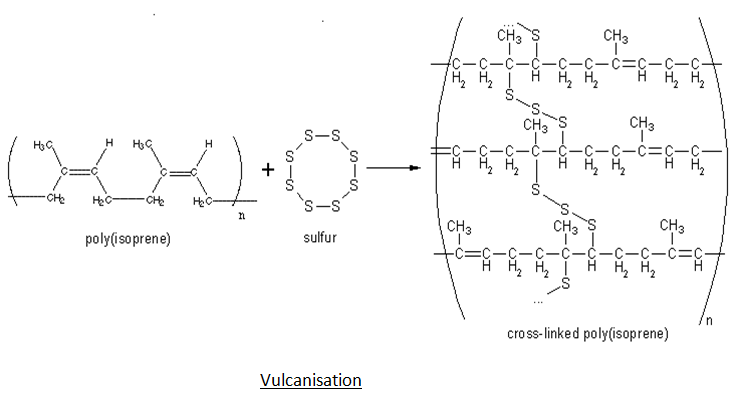
Silicone rubber s higher heat resistance makes it suitable for casting metals with melting points of less than 315 f 157 c such as pewter and tin directly into the mold. Silicone rubber also has a higher vulcanizing temperature than natural rubber 330 f 165 c to 350 f 177 c compared to 310 f 154 c for natural rubber. Natural rubber is made from plant material. Once the rubber is vulcanized it is a thermoset.
Pure rubber once vulcanised can not be melted or the tires on your car would melt under heavy braking. In rubber manufacture different chemical substances are added to the polymer mass. These items are made either by casting latex or immersing a mold into it. Trade names include perbunan nipol krynac and europrene this rubber is unusual in being resistant to oil fuel and other chemicals.
Most rubber in everyday use is vulcanized to a point where it shares properties of both. Macromolecular chemistry and physics 2013 214 8 912 923. Some of these are effective in production for example vulcanization accelerators while others play an important role in the finished product e g. Nbr is used in the automotive and aeronautical industry to make fuel and oil handling hoses seals grommets.
Among the many uses of rubber is for the manufacturing of tires footwear and gloves.